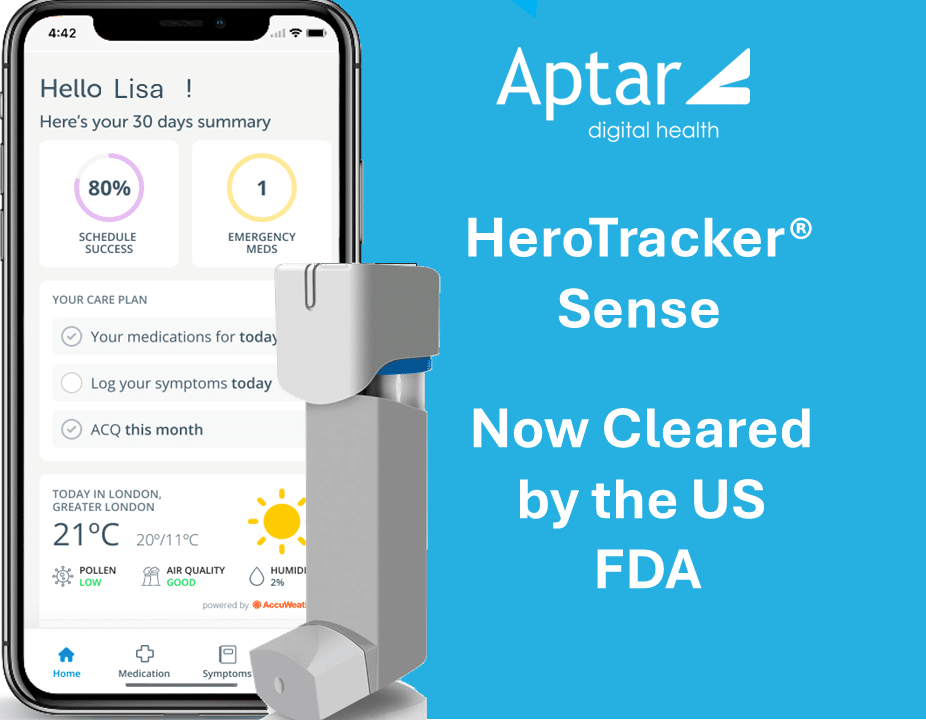
OCTOBER 16, 2025
Aptar Digital Health announces that the U.S Food and Drug Administration (FDA) has granted 510(k) clearance for HeroTracker® Sense as a Class II medical device. This milestone recognizes HeroTracker® Sense – a Bluetooth-enabled sensor as an innovative connected add-on for pressurized Metered Dose Inhalers (pMDIs), transforming traditional inhalers into smart, data-driven devices for patients and healthcare professionals.
HeroTracker® Sense supports users in managing their respiratory health through comprehensive medication and symptom management. The platform delivers real-time usage tracking, personalized reminders and educational resources via an intuitive mobile application, encouraging behavior change and adherence to prescribed therapies. By enabling patients to monitor inhaler techniques and receive instant feedback, the platform aims to support improved respiratory health.
Learn more about our approach in respiratory care.