Companion Apps, or digital Patient Support Programs (PSPs), are designed to improve patient onboarding and adherence to a specific treatment. These digital tools are typically non-regulated, non-interventional, and highly user-centric, providing patients with personalized support and resources to help them navigate their treatment journey.
Digital PSPs can be developed as stand-alone solutions or embedded as specific modules into existing digital health products, such as Aptar Digital Health MigraineBuddy.
By providing patients with easy access to information about their condition and treatment, as well as tools to track their symptoms and progress, digital PSPs can help patients feel more empowered and engaged in their care.
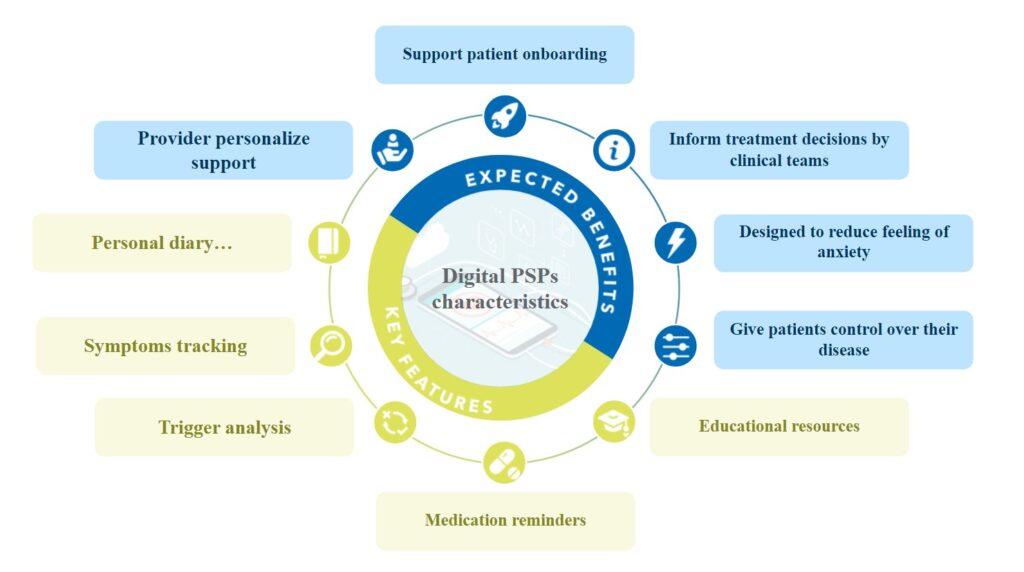
One of the key benefits of digital PSPs is their ability to provide patients with personalized support during the early phases of their treatment journey. The first few weeks or months of starting a new therapy can be a critical time for patients, as they may be experiencing new symptoms or side effects, and may be unsure about what to expect with an increasing feeling of anxiety. Digital PSPs can help bridge this gap by providing patients with on-demand access to educational resources, medication reminders, and other supportive features that can help them feel more confident and in control of their treatment.
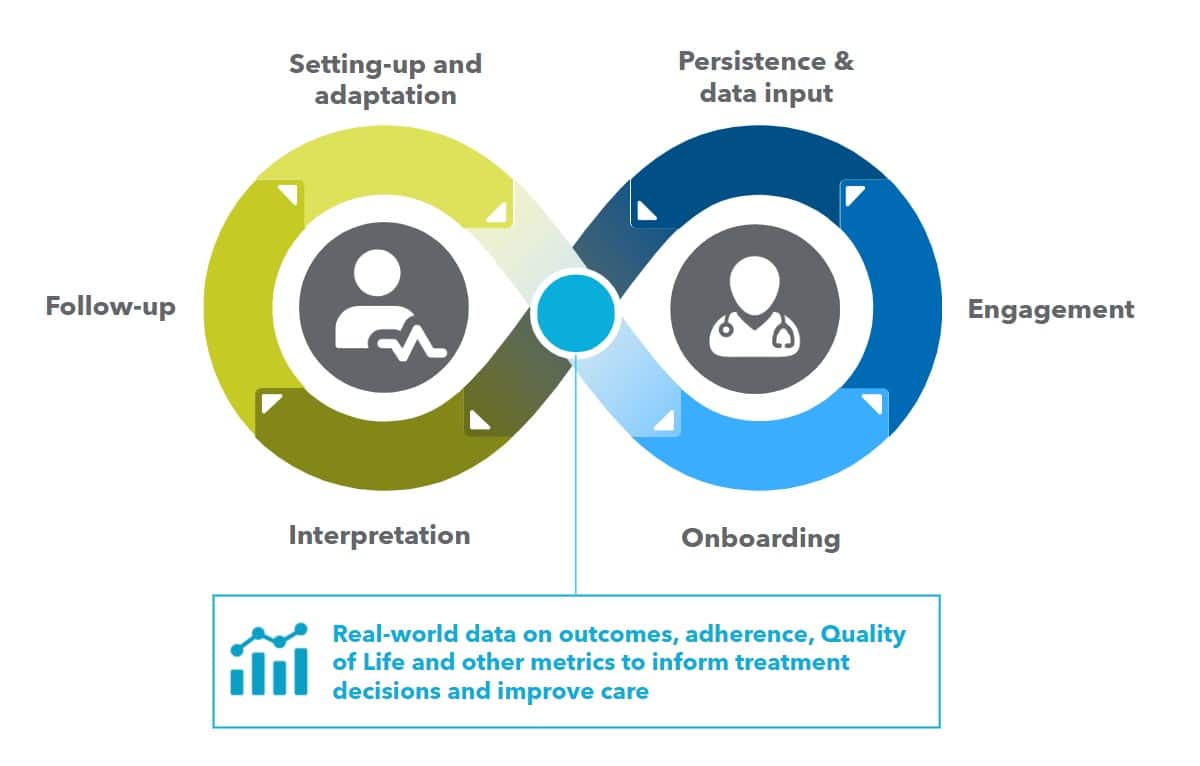
In addition to contributing to patient onboarding and adherence, digital PSPs can also provide valuable insights and data for healthcare providers and pharmaceutical companies. By collecting real-world data on patient outcomes, medication adherence, and other relevant metrics, digital PSPs can help inform treatment decisions and improve patient care.
Software-as-a-Medical Device (SaMD) is a type of regulated software designed to address specific pain points in the patient journey, from early disease identification Digital Diagnostics – DDx) to routine treatment. SaMD is a medical device and is subject to regulatory oversight.
SaMD solutions can range from mobile apps that help patients manage their medication to complex algorithms that analyze patient data to provide personalized treatment recommendations. These solutions are often tied to a specific drug or therapy and are intended to be used in conjunction with that treatment.
One of the key specificities of SaMD is the need for clinical evidence to support their use. Because SaMD is a medical device, it must be validated through clinical trials and other studies to demonstrate its safety and efficacy. Building a robust SaMD is a demanding exercise with level of clinical rigor and regulatory excellence.
Another important aspect of SaMD is its regulation. Depending on the intended use and risk level of the software as well as the targeted markets, SaMD may be subject to different regulatory requirements. This can include compliance with quality management systems, cybersecurity requirements, and other regulations specific to medical devices.
Despite these challenges, SaMD has the potential to transform the way we approach treatment and care. By leveraging the power of software and data analytics, SaMD can help providers and patients make more informed decisions, improve adherence, and optimize treatment outcomes.
Moreover, SaMD can provide new opportunities for pharmaceutical companies to differentiate their products and deliver more value to patients. By developing SaMD solutions that are tightly integrated with their drug assets, pharmaceutical companies can create a more comprehensive and personalized treatment experience for patients.
Disease Management Platforms are a comprehensive suite of products and services that aims to address most patients’ needs in a single solution.
The platform is designed to provide an end-to-end solution that supports patients throughout their treatment journey, and improve their overall health outcomes.
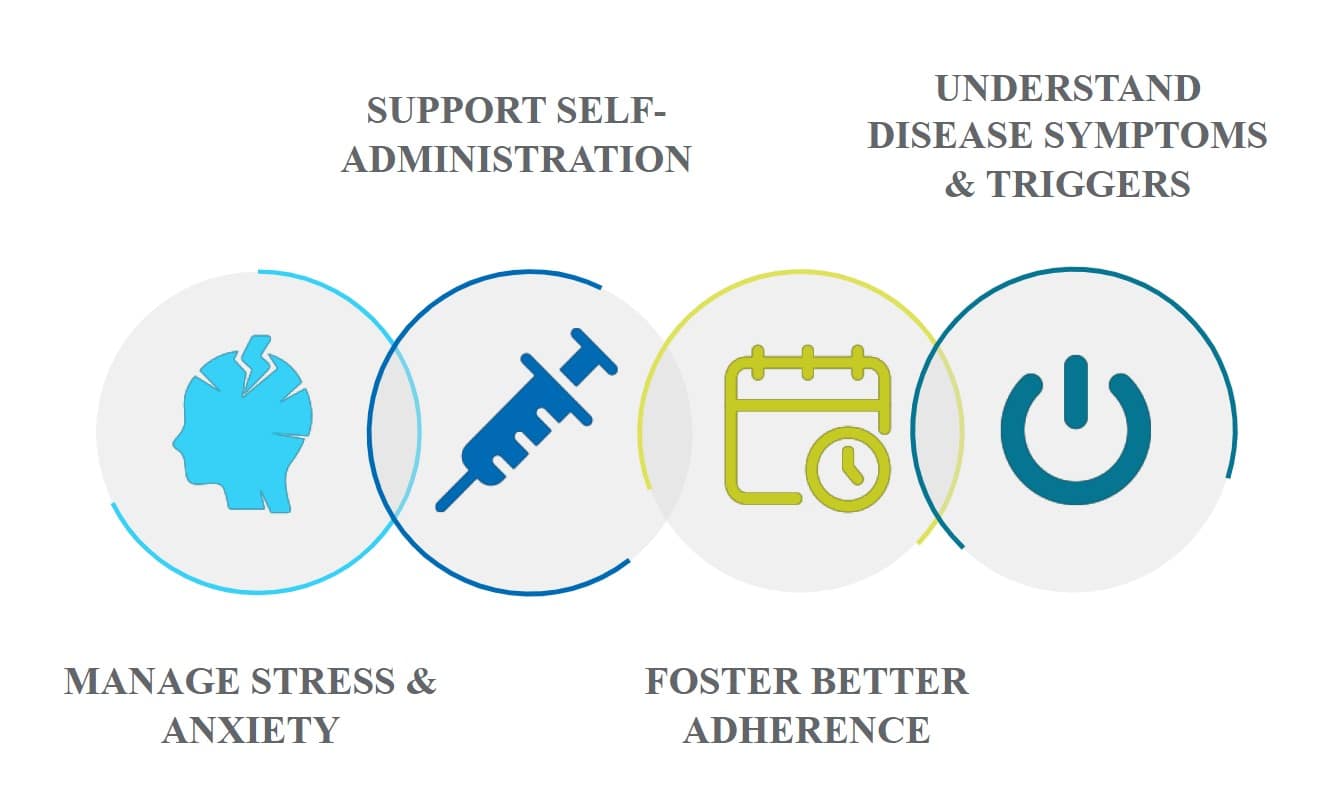
Pain points of interest can include (but are not limited to): support to self-administration, manage stress and anxiety related to disease and/or treatment, improve adherence over time, help identify and understand triggers for disease symptom.
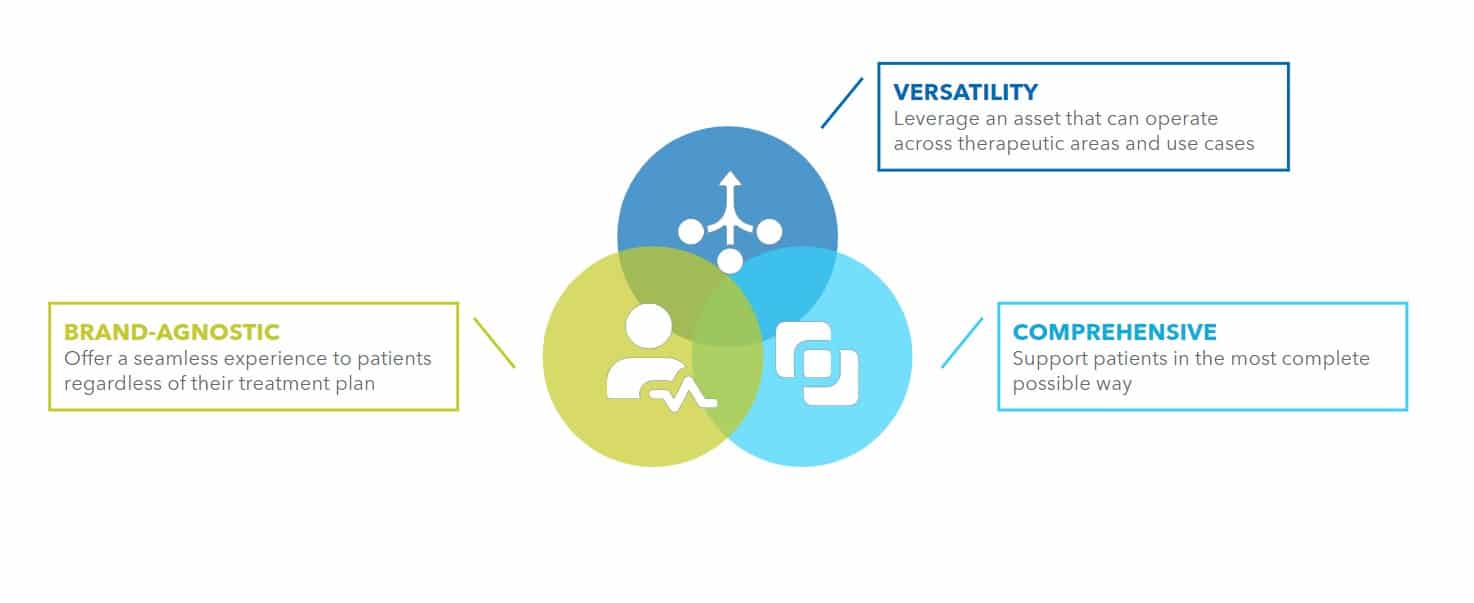
One of the key benefits of a Disease Management Platform is its versatility. Depending on the specific needs of the patient population, the platform can include a mix of software and hardware components such as administration devices, wearables, and sensors. This flexibility allows the platform to be customized to meet the unique needs of each patient, providing a personalized and integrated care experience.
Another important aspect of a Disease Management Platform is its ability to be agnostic. The platform can be used to support patients regardless of the specific treatment they are receiving. This approach provides users with a consistent and seamless care experience, independently of their therapy.
The ability to target multiple pain points is another key advantage. By addressing a broad range of needs, the platform can improve patient outcomes and quality of life in a more comprehensive way instead of offering multiple “point-solutions” that create confusion at user level.
The regulatory status of a Disease Management Platform can vary depending on the specific components that are used and the intended claims. However, given the platform’s focus on improving patient outcomes and quality of life, it may be eligible for reimbursement from health systems.
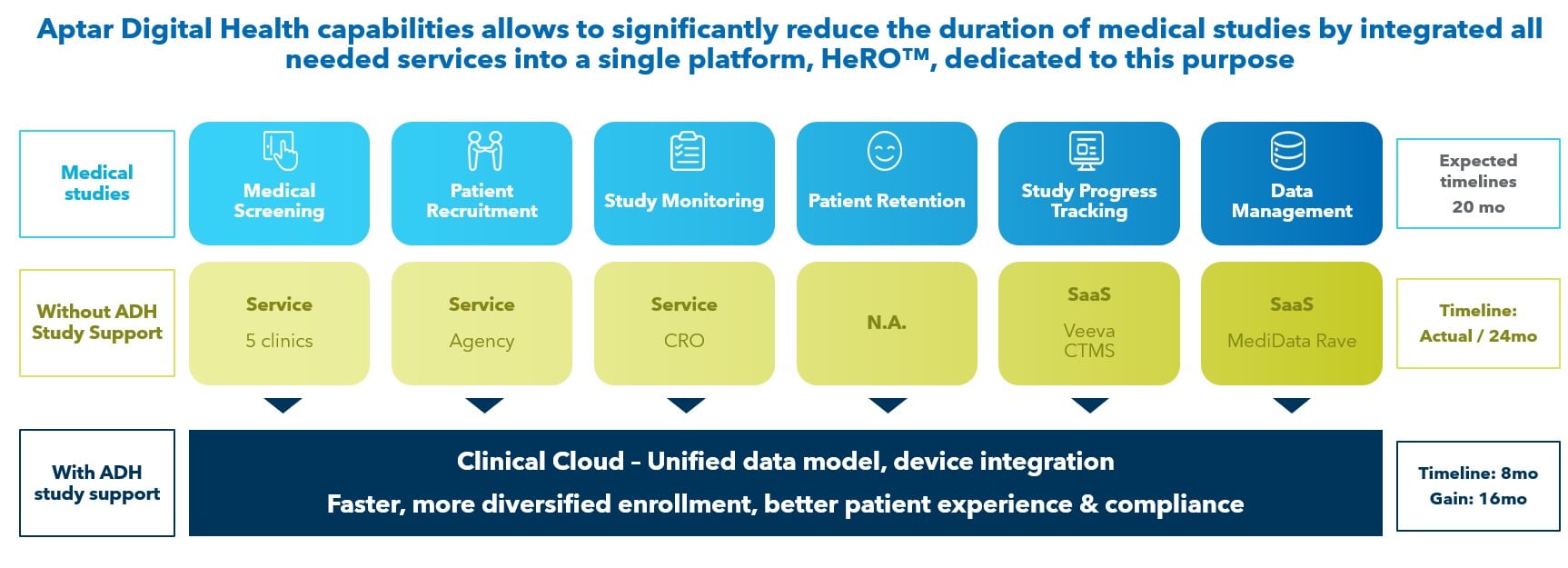
In addition to accelerating the clinical trial process, our study support services also help to derisk execution. This can improve the quality of data collected during the study, leading to more reliable and accurate results.
Our study support services offer significant value to pharmaceutical companies in both clinical trial and real-world evidence settings. By leveraging our digital health capabilities, we can help pharma companies to accelerate and derisk the execution of studies, improving efficiency, accuracy, and predictability. This can ultimately lead to better patient outcomes and improved treatment options.
"*" indicates required fields
* to process your contact request. If you do not fill them in, your request cannot be processed. To find out more about the use of your personal data and how to exercise your rights, please consult the Privacy Policy